- Research Offices
- OHSP
- Student-led Activities and Research
- Student-led Research FAQs Pre-Knowledge Base
FREQUENTLY ASKED QUESTIONS ABOUT STUDENT-LED ACTIVITIES & RESEARCH: To find an answer, click on an FAQ below. To return to the FAQ list, click again on the FAQ or "v" symbol. For many other FAQs that apply to all FSU research that may involve respondents or study participants, visit this main FAQs page (for international research, see our newest FAQ #12, under Specific Questions).
�
�
- An IRB is a committee composed of scientists and non-scientists (e.g., professionals as well as laypersons) that may be affiliated with an institution or organization, as well as persons not affiliated with these institutions or organizations (referred to as IRB members), that review and provide ethical and regulatory oversight of human research that is proposed and conducted by other persons affiliated with those institutions or organizations. IRB members are expected to have the background, experience and expertise required to ascertain the ethical and regulatory acceptability of proposed and ongoing research, primarily to help ensure the protection of the rights and welfare of individuals who serve as human research participants (human subjects) in research.
- The Florida State University (FSU) has an IRB. Institutions like FSU which receive federal funds to conduct human research are required by federal law to have an IRB. Federal regulations provide the FSU IRB with the authority to approve, require modifications in (to secure approval), or disapprove all research activities covered applicable law and FSU policy. Furthermore, while research that has been approved by the FSU IRB may be subject to further review and approval or disapproval by FSU officials, these officials may not approve the research if it has not been approved by the FSU IRB.
- The IRB is not permitted by law to retroactively review human research.
- Only human research that complies with applicable law and policy is approved by the FSU IRB.
- Proposed human research that does not comply with applicable law and/or policy or the IRB’s requirements will be returned to the study team until the proposed research meets the IRB's criteria and conditions for approval.
- Previously approved research which is determined to violate applicable law and policy and the IRB conditions for approval will be suspended and may be terminated. Violations may require reporting to FSU officials, federal regulatory agencies and study sponsors. Subsequent proposed and any on-going research conducted by the study team may be subject to heightened review scrutiny and restrictions.
- All FSU students that plan to conduct activities that may involve interactions or interventions with individuals to collect information or biospecimen, or use of individuals' information or biospecimens (even without interaction or intervention) must submit a protocol in the University's Research Administration Management Portal (RAMP) Institutional Review Board (IRB) online electronic protocol management system ("RAMP IRB"). OHSP will triage the protocol and conduct pre-review before routing the activity for any further or necessary IRB review.
- Any student who is not sure about whether their activity will undergo further IRB review may, in lieu of a full IRB protocol, first submit in RAMP IRB a completed HRP-503d - Template Determination Form. OHSP will review the form and related materials and let you know whether further IRB review is required and if so, what additional information and materials must be submitted. The HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs. See this Request a Determination video tutorial to see how to submit the HRP-503d form in RAMP IRB.
- Students should carefully look over the HRP-503d - Template Determination Form BEFORE contacting OHSP with questions to be certain that they have gathered all relevant information necessary in order to save time and effort.
Generally, advance review and approval is required of any College of Medicine (COM) medical student-led activity that may, for a research purpose, involve individuals as respondents or study participants when the activity will include (1) interaction or intervention with individuals to collect their information or biospecimens, or (2) collection or use of individuals' information or biospecimens (e.g., secondary use of previously collected information, even when students will have no interaction or intervention with the individuals). However, an exception may be made for COM medical student activities conducted solely for purposes of their clerkships. Visit our Medical Students web page for additional and specific information.

- The following typical student-led activities do not usually require OHSP or IRB review. These are examples only; carefully review the criteria:
- Class projects: Many class projects are intended to teach students research-related skills and provide opportunities to practice research methods such as design; sampling; literature searches; observation, interview, or survey techniques; and data analyses. In some cases, student-led activities to acquire, test or refine research-related skills may include a research component. Class projects involving student-led activities intended exclusively for instructional purposes and not intended to develop or contribute to generalizable knowledge do not require further OHSP or IRB review and approval.
- Oral history, journalism, biography, literary criticism, legal research, and historical scholarship, including the collection and use of information that focus directly on the specific individuals about whom the information is collected AND from which activity or analyses of the collected information there is no intent to draw conclusions or generalize findings beyond the specific individuals involved in the activity. However, if the activity or analyses of collected information will be used to document and draw conclusions about the individuals’ collective experiences, inform policy or otherwise generalize findings beyond the specific individuals involved, then these activities will require OHSP or IRB review.
- If your activity is atypical or if you are not sure if your student-led activity will require OHSP or IRB review, submit in RAMP IRB a completed HRP-503d - Template Determination Form and related materials; OHSP will review the form and related materials and let you know whether further IRB review is required and if so, what additional information and materials must be submitted. The HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs. See this Request a Determination video tutorial to see how to submit the HRP-503d form in RAMP IRB.
- RAMP IRB is accessible within the myFSU portal as an icon under the myFSU Links section, and you must therefore have a valid FSU user credential. The system is designed to be part of the single sign-on process, allowing FSU student users to conveniently access RAMP IRB along with other systems within a single platform using one set of login credentials. Use any of the means below to access the RAMP IRB system to submit your study:
- Sign on to the myFSU portal https://www.my.fsu.edu/portal and click on the RAMP icon--

-
- Visit https://myramp.research.fsu.edu; or,
- Click on the link from an auto-generated email from ramp-irb@fsu.edu
- Video tutorials are available to assist you with using RAMP IRB and navigating a study submission workspace. Tutorials last just a few minutes and include video captures of RAMP IRB workspaces. To access the tutorials go here.
- For written step-by-step instructions, refer to the Researcher's Guide to RAMP IRB, or to the OHSP Researchers Training slides available in RAMP IRB Help Center.
- For other questions about using RAMP IRB, including obtaining assistance with using RAMP IRB, visit this general RAMP IRB FAQ web page, also available at: https://ramp.research.fsu.edu/faqs/irb/
- Generally all documents and materials used to plan and conduct your activity must be submitted in RAMP IRB for review, and the use of our templates is required.
- Common documents and materials include, for example, the protocol (e.g., the activity or study's objectives, hypotheses, plan, methods, sample, settings etc.), actual measures and instruments and anything that respondents or study participants will be asked to read, complete, watch or use; recruitment fliers and messages; consent forms; and outside approvals. Other materials may also be required depending upon planned activities.
- The RAMP IRB SmartForms will include instructions and directions for you to follow for submitting and uploading related information, documents and materials. Pay particular attention to any ? Help buttons located in each setting or next to each field, as these provide detailed instructions and links.
- Studies that involve interactions or interventions with respondents or study participants will require a consent process through which you will recruit and ask prospective subjects for their permission to involve them in your activity.
- The OHSP and IRB provide a wide selection of protocol and consent templates for your use; these templates are tailored to different types of studies (biomedical; non-biomedical, such as social, behavioral or educational studies) and study populations (e.g., children, adults, limited English language proficiency). These templates may change to reflect current regulatory requirements. Use only the templates available in RAMP IRB under the IRB, Library and Templates tabs. OHSP and IRB criteria for review follow the templated protocols; if you do not use the current and correct templates your submission will be returned to you.
- Also, if you will only obtain secondary (previously collected) data but not have any interaction with respondents or study participants, then you must provide a list of all data variables or a data dictionary (highlighting or indicating what data variables will be obtained). If you will directly or indirectly interact with respondents or study participants (i.e., surveys, interviews, focus groups, collect any other data or information, conduct in-person, face-to-face, virtually, remotely or by any other means), then you must provide ANY and ALL material that will be seen, heard, read or completed by or for these individuals, including recruitment fliers or messages, videos, images, and data collection instruments and measures. Sample measures or links to materials do not suffice for IRB finalization.
- Save yourself time and effort! Carefully look over our HRP-308 Worksheet - Pre-Review for the list of information and materials that OHSP will check in order to deem research as IRB "review ready". Submissions that are missing information and materials will be returned to the study team for correction. HRP-308 is accessible in RAMP IRB, under the IRB, Library and Worksheets tabs (scroll down the HRP-308).
- Also refer to pages 14-15 of the Researcher's Guide, Checklist of Information to Attach, as a handy reference list of these materials and information.
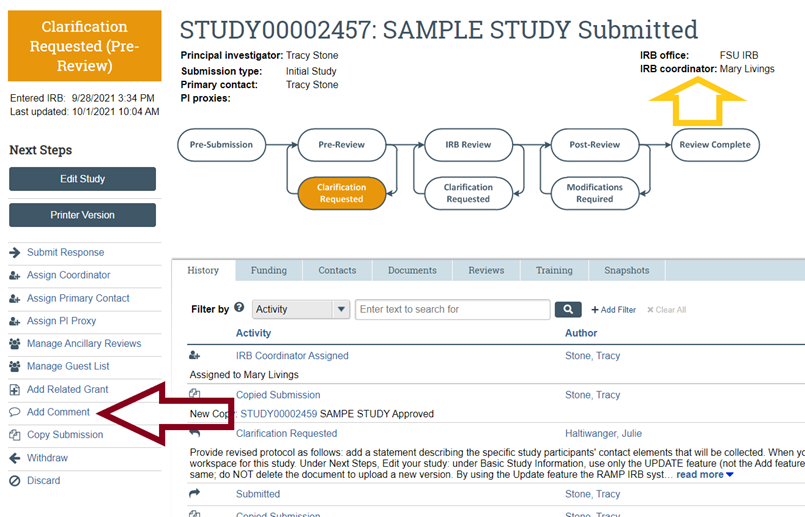
- Always log in to RAMP IRB and view your submission’s workspace to check the status of your submission. The workflow diagram and the History tab entries will indicate your submission status. In the figure below, the example workflow diagram indicates that your activity is in the Pre-Submission state (the activity has not yet been submitted to OHSP and IRB for their review), and under the History tab it shows that you have created (but not submitted) your activity.

- For a more detailed explanation about using the workflow diagram to check the status of your submission, click on this RAMP IRB workflow explainer.
- Be sure to follow instructions on the Final Page in the RAMP IRB SmartForm for your activity, which states that you must “click Submit” in order for your activity to ever enter the IRB review queue. With many thousands of FSU faculty, staff and students, the IRB does not search out for activities in the Pre-Submission stage to follow up for reasons in delay as there may be a myriad of reasons why faculty, staff and students maintain their activities in the Pre-Submission state.
- Note that only these individuals who are authorized to actually submit to the IRB (i.e., Principal Investigators (PIs) or PI proxies, i.e., the student that will led the activity) will be able to see the “Submit” button. If you are not the PI or PI Proxy for the activity, then you will not be able to see nor execute the “Submit” feature. The study PI or PI Proxy needs to log in; they will see the “Submit” button above the “Assign Primary Contact” button. The PI has the option to “Assign PI Proxy” so therefore they can click Submit or they assign another team member as PI Proxy; that team member will then have the authority to click “Submit” on the PI’s behalf.
- Special note if you receive the notification “Response Time Exceeded”: these notifications are only intended as a courtesy to alert you that it has been 14 days since clarification was requested on your submission and our office has not yet received your response. You may still make or submit edits and take the time that you need to do (unless you have been specifically told about a deadline for re-submission; check your correspondence from IRB). You will continue to receive these notifications until you take the required action noted in the Clarification Requested instructions.
- Refer to our Human Research Review web page to learn about OHSP/IRB turn-around times. The referenced anticipated turn-around times apply only to submissions that are complete (required forms, materials, information and requested clarifications have been submitted or completed, including completion of the required CITI human subjects training for all study staff). Carefully review ALL requirements before submitting otherwise you will risk needless further delay that could have been anticipated and avoided.
- If you have additional questions about the status of your submission, contact OHSP by submitting a Comment in your study's RAMP IRB workspace, under Next Steps; there, in response to the question about who should receive the email notification about your comment, select "IRB Coordinator." This will help to ensure that the IRB Coordinator assigned to your submission will receive a notification that your comment has been posted in your RAMP IRB workspace. Alternatively and if you have not yet submitted your activity (i.e., your activity is till in the Pre-Submission status) or an IRB Coordinator has not yet been assigned to your submission (i.e., no name is listed next to "IRB Coordinator"), contact OHSP or refer to the contact information in the left panel, and your question will be routed to OHSP staff for follow-up with you directly.
- A faculty advisor may be your major professor or professor for the course for which you are conducting the activity; the faculty director for the academic program for which you are conducting the activity; your thesis advisor or a member of your dissertation committee for which your activity will be used to satisfy a thesis or dissertation requirement; or other faculty mentor with whom you are working on your activity.
- The faculty advisor must be a member of the FSU faculty, and have a valid FSU user credential in order to access RAMP IRB.
- A faculty advisor may be expected to:
- meet with the student to monitor the activity's progress;
- be available to assist the student in solving problems should these arise;
- advise the student about the student’s responsibilities; and,
- arrange for an alternate faculty advisor as may be needed.
FSU student researchers:
- CITI training completion documentation is synced with RAMP IRB each evening through a nightly data transfer. You should wait for the next day after CITI training course completion to add yourself to a RAMP IRB submission.

- Your CITI profile must:
- Affiliate with FSU, and
- Be accurate, including using your official FSU email address as the primary email.
- The data transfer feature is a courtesy to the FSU community, and made possible by separate arrangement between the 2 proprietary vendors that support CITI and RAMP IRB. However, if your CITI training completion documentation does not transfer, then you will be required to upload the CITI training completion documentation within the RAMP IRB submission workspace.
- Refer to the Investigator Manual for information about CITI human subjects research training requirements or visit our website’s training page for step-by-step instructions on how to enroll in the correct training: https://www.research.fsu.edu/research-offices/ohsp/investigator-resources/citi-training-requirements/. Only the specified training will suffice for IRB review purposes.
Non-FSU activity or study team members:
- You must provide the FSU Principal Investigator with a copy of your CITI training completion documentation, and the FSU Principal Investigator must upload the copy in other study documents within the RAMP IRB submission workspace.

- This will depend upon several key factors: if your submission (1) is complete, is accompanied by required documents, and satisfactory responses have been provided to any requested clarifications; (2) formally documented by OHSP as exempt and not subject to complete IRB review; (3) will require IRB committee (at a scheduled monthly meeting) or non-committee (subcommittee, also referred to expedited, for which review may be done at any time) review; and (4) requires any other outside or ancillary review (e.g., another institution's IRB reviews; safety reviews for use of hazardous materials or radiation-emitting devices; ethics or conflicts of interest reviews) outside of the OHSP or IRB. With the above caveats, particularly item (1) above:
- Exempt studies or activities that involve only use of de-identified information may receive clearance within 2 weeks or 10 business days, often less (surveys, focus groups and interviews generally fall into this review pathway);
- Expedited or non-committee (outside a scheduled monthly meeting and on a rolling basis) reviews may be completed within 4 weeks (interventions that only involve minimal risk, use of identifiable but benign information, involve respondents or study participants who are neither minors nor vulnerable, and don't involve other institutions, generally fall into this expedited review pathway);
- Committee (at a scheduled once-monthly meeting) reviews may be completed within 6 weeks (interventions that involve a minor increase over minimal risk or pose greater than minimal risk, use of identifiable information the release of which may pose risks to individuals about whom the information pertains, clinical trials, studies involving use of FDA-regulated products, multisite research, and involve vulnerable study participants, will often fall into this convened review pathway)
- IF outside or ancillary reviews by other FSU offices or non-FSU institutions are required, this will add time before an activity or study may begin, so plan accordingly, such as obtaining these reviews before or while your activity undergoes IRB review.
- Review turn-around is also subject to other factors, such as IRB reviewer availability or workload, complex activities (e.g., studies involving use of unapproved drugs, medical devices and other products subject to additional federal laws; agreements or arrangements with other outside agencies, institutions or sponsors) or sponsor requirements.
- Save yourself time and effort! Carefully look over our HRP-314 Checklist - Criteria for Approval for the list of federal regulatory criteria that the IRB must apply to their review before granting approval. Submissions that are missing information and materials necessary for the IRB to indicate criteria have been satisfied will be returned to the PI and/or team for correction. HRP-314 is accessible in RAMP IRB, under the IRB, Library and Checklists tabs.
-
If you leave FSU, you must either close your activity or study, or (if the activity is to remain on-going at FSU) modify your activity to designate another FSU individual as the Principal Investigator (PI).
- To close your activity in RAMP IRB, follow these steps: create a continuing review submission, complete the Continuing Review/Study Closure Information items as applicable (the first 4 milestones must be checked), acknowledge that the activity will be closed, discard any open follow-on submissions related to the activity, complete the remaining items, attach any supporting documents as may be needed, then select Save, Finish and under Next Steps select Submit. Your Study Closure submission will move into the queue for review and further handling.
- To designate another FSU individual as PI, follow these steps: create and submit a modification, indicating the indicating the scope as “other parts of the study,” providing modification information (e.g., status of study enrollment), and the explain that you will designate a new FSU PI. On the Basic Study Information page, under Local Principal Investigator, indicate the new FSU PI, make any necessary changes to this or other pages, and answer Yes or No to the Additional Requirement page about the PI’s student status (and if Yes, indicate the name of the faculty advisor or major professor), then Finish and Submit (Under Next Steps) the modification. Your modification will (after faculty advisor review for a new FSU student PI, if applicable) move into the queue for review and further handling.
- Note that if another FSU individual is designated as PI, you may remain on the team provided that your new institution (if you have a new institution) is aware of your continuing role on the activity, and you make any necessary arrangements with your new institution to rely on the FSU IRB for continuing oversight.
- For additional instructions about continuing review and modification submissions, refer to the to the Researcher's Guide to RAMP IRB, or to the OHSP Researchers Training slides available in RAMP IRB Help Center.
- To close your activity that was never submitted within RAMP IRB, complete and submit to OHSP the closure form located here on the OHSP web site.
- Refer first to our main FAQs intended for the entire FSU research community; many more FAQs are posted there and may help to answer one or more of your questions.
- If you have additional questions about the status of your submission not listed above, contact OHSP using the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your submission workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow); there, in response to the question about who should receive the email notification about your comment, select "IRB Coordinator." This will help to ensure that the IRB Coordinator assigned to your submission will receive a notification that your comment has been posted in your RAMP IRB workspace.

- Alternatively and if you have not yet submitted your activity, contact OHSP.


